Learning Outcomes
- The Atomic Mass Of An Element Is Equal To __
- What Is The Atomic Mass
- The Atomic Mass Of An Element Is Equal To The:
- Define atomic and mass numbers.
- Determine the number of protons, neutrons, and electrons in an atom.
- Identify the charge and relative mass of subatomic particles.
- Label the location of subatomic particles in the atom.
- Define isotope.
- Write the isotopic symbol of an atom.
- Explain the concept of average atomic mass.
- The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number.
- Element X has two isotopes. If 72.0% of the element has an isotopic mass of 84.9amu and 28.0% has an isotopic mass 87.0amu, the average atomic mass of element X is numerically equal to.
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. Since neutrons do not affect the charge, the number of neutrons is not dependent on the number of protons and will vary even among atoms of the same element.
A relative atomic mass is the ratio of the average mass per atom of an element from a given sample to 1/12 the mass of a carbon-12 atom. We find the relative atomic mass of a sample of an element by working out the abundance-weighted mean of the relative isotopic masses.
Atomic Number
The atomic number (Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons.
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass numberis defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number | Protons | Neutrons | Electrons | Mass Number |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 7 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 11 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12 |
View animations showing the atomic structure of the first 11 elements on the periodic table at http://web.visionlearning.com/custom...imations.shtml
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
[text{Number of neutrons} = text{mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation using the atomic number and the mass number. Both are written before the chemical symbol, with the mass number written as a superscript and the atomic number written as a subscript. The chromium atom discussed above would be written as:
[ce{^{52}_{24}Cr}]
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The above atom would be written as chromium-52, with the mass number written after the name. The atomic number does not have to be included because all atoms of chromium have the same number of protons but can vary in the atomic mass.
Isotopes
As stated earlier, not all atoms of a given element are identical. Specifically, the number of neutrons in the nucleus can vary for many elements. As an example, naturally occurring carbon exists in three forms, which are illustrated in the figure below.
Each carbon atom has the same number of protons (6), which is equal to its atomic number. Each carbon atom also contains six electrons, allowing the atom to remain electrically neutral. However, the number of neutrons varies from six to eight. Isotopesare atoms that have the same atomic number but different mass numbers due to a change in the number of neutrons. The three isotopes of carbon can be referred to as carbon-12 (left( ce{^{12}_6C} right)), carbon-13 (left( ce{^{13}_6C} right)), and carbon-14 (left( ce{^{14}_6C} right)). Naturally occurring samples of most elements are mixtures of isotopes. Carbon has only three natural isotopes, but some heavier elements have many more. Tin has ten stable isotopes, which is the most of any known element. The nucleus of a given carbon atom will be one of the three possible isotopes discussed above.
While the presence of isotopes affects the mass of an atom, it does not affect its chemical reactivity. Chemical behavior is governed by the number of electrons and the number of protons. Carbon-13 behaves chemically in exactly the same way as the more plentiful carbon-12.
Size of Atoms
The graphite in your pencil is composed of the element carbon, a nonmetal. Imagine taking a small piece of carbon and grinding it until it is a fine dust. Each speck of carbon would still have all of the physical and chemical properties of carbon. Now imagine that you could somehow keep dividing the speck of carbon into smaller and smaller pieces. Eventually, you would reach a point where your carbon sample is as small as it could possibly be. This final particle is called an atom.
Atoms, as you probably know, are extremely small. In fact, the graphite in an ordinary pencil contains about (5 times 10^{20}) atoms of carbon. This is an almost incomprehensibly large number. The population of the entire Earth is about (7 times 10^9) people, meaning that there are about (7 times 10^{10}) times as many carbon atoms in your pencil as there are people on Earth! For this to be true, atoms must be extremely small. We can only see atoms with a modern instrument called a scanning tunneling microscope. (www.nobelprize.org/educationa...opes/scanning/)
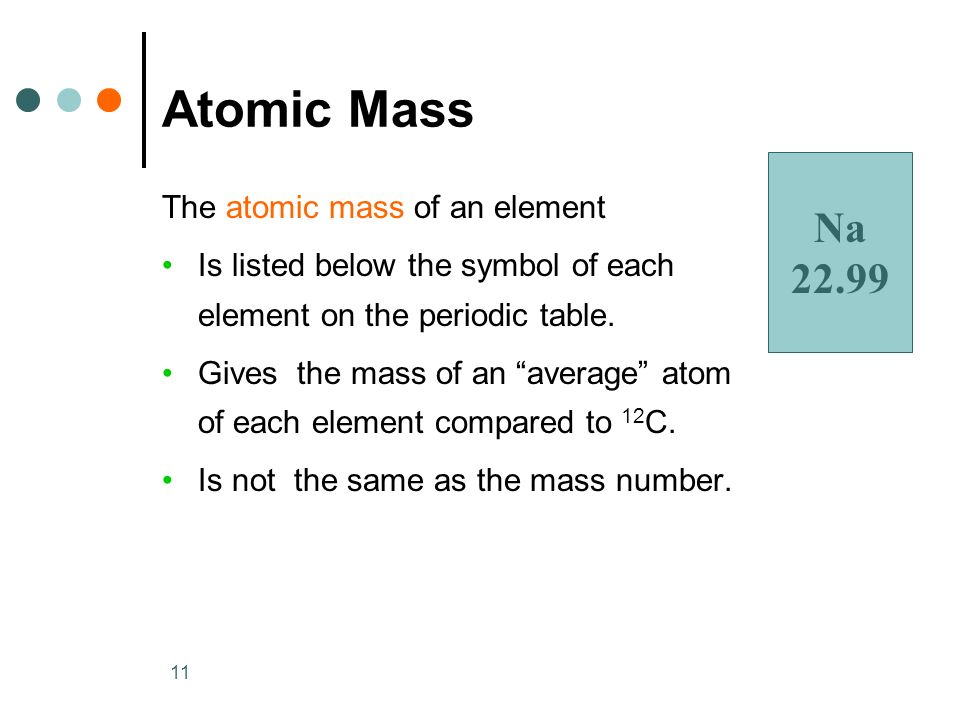
Atomic Mass
The masses of individual atoms are very, very small. However, using a modern device called a mass spectrometer, it is possible to measure such miniscule masses. An atom of oxygen-16, for example, has a mass of (2.66 times 10^{-23} : text{g}). While comparisons of masses measured in grams would have some usefulness, it is far more practical to have a system that will allow us to more easily compare relative atomic masses. Scientists decided on using the carbon-12 nuclide as the reference standard by which all other masses would be compared. By definition, one atom of carbon-12 is assigned a mass of exactly 12 atomic mass units (left( text{amu} right)). An atomic mass unitis defined as a mass equal to one twelfth of an atom of carbon-12. The mass of any isotope of any element is expressed in relation to the carbon-12 standard. For example, one atom of helium-4 has a mass of (4.0026 : text{amu}). An atom of sulfur-32 has a mass of (31.972 : text{amu}).
The carbon-12 atom has six protons and six neutrons in its nucleus for a mass number of 12. Since the nucleus accounts for nearly all of the mass of the atom, a single proton or single neutron has a mass of approximately (1 : text{amu}). However, as seen by the helium and sulfur examples, the masses of individual atoms are not quite whole numbers. This is because an atom's mass is affected very slightly by the interactions of the various particles within the nucleus and also includes the small mass added by each electron.
As stated in the section on isotopes, most elements occur naturally as a mixture of two or more isotopes. Listed below (see table below) are the naturally occurring isotopes of several elements along with the percent natural abundance of each.
| Element | Isotope (Symbol) | Percent Natural Abundance | Atomic mass (left( text{amu} right)) | Average atomic mass (left( text{amu} right)) |
|---|---|---|---|---|
| Hydrogen | (ce{^1_1H}) | 99.985 | 1.0078 | 1.0079 |
| (ce{^2_1H}) | 0.015 | 2.0141 | ||
| (ce{^3_1H}) | negligible | 3.0160 | ||
| Carbon | (ce{^{12}_6C}) | 98.89 | 12.000 | 12.011 |
| (ce{^{13}_6C}) | 1.11 | 13.003 | ||
| (ce{^{14}_6C}) | trace | 14.003 | ||
| Oxygen | (ce{^{16}_8O}) | 99.759 | 15.995 | 15.999 |
| (ce{^{17}_8O}) | 0.037 | 16.995 | ||
| (ce{^{18}_8O}) | 0.204 | 17.999 | ||
| Chlorine | (ce{^{35}_{17}Cl}) | 75.77 | 34.969 | 35.453 |
| (ce{^{37}_{17}Cl}) | 24.23 | 36.966 | ||
| Copper | (ce{^{63}_{29}Cu}) | 69.17 | 62.930 | 63.546 |
| (ce{^{65}_{29}Cu}) | 30.83 | 64.928 |
For some elements, one particular isotope is much more abundant than any other isotopes. For example, naturally occurring hydrogen is nearly all hydrogen-1, and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in substantial quantities. Chlorine (atomic number 17) is yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would get approximately 36.
[frac{34.969 + 36.966}{2} = 35.968]
As you can see, the average atomic mass given in the last column of the table above is significantly lower. Why? The reason is that we need to take into account the natural abundance percentages of each isotope in order to calculate what is called the weighted average. The atomic massof an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. The average atomic masses are the values we see on the periodic table.
[0.7577 left( 34.969 right) + 0.2423 left( 36.966 right) = 35.453]
The weighted average is determined by multiplying the percent of natural abundance by the actual mass of the isotope. This is repeated until there is a term for each isotope. For chlorine, there are only two naturally occurring isotopes so there are only two terms.
Supplemental Resources
- Elements and Atoms: http://www.youtube.com/watch?v=IFKnq9QM6_A
- Introduction to the Atom: http://www.youtube.com/watch?v=1xSQIwWGT8M
- Atomic History - A Brief Discovery: http://www.pbs.org/wgbh/nova/diamond...dehistory.html
- All About Atoms: http://education.jlab.org/atomtour/index.html
- Build and Atom: http://phet.colorado.edu/en/simulation/build-an-atom
- Molecular Workbench - Atomic Structure: http://workbench.concord.org/databas...vities/47.html
- See Inside a Diamond: http://www.pbs.org/wgbh/nova/diamond/insidewave.html
- Isotopes and Atomic Mass: http://phet.colorado.edu/en/simulati...nd-atomic-mass
- Atomic Structure: freezeray.com/flashFiles/atomcStructure.htm
- Atom Builder: freezeray.com/flashFiles/atomBuilder.htm
- Tennis Ball Isotopes: http://www.youtube.com/watch?v=oLnuXpf4hsA
- Element Math Game: http://education.jlab.org/elementmath/index.html
- Atoms and Matter Crossword Puzzle: http://education.jlab.org/sciencecro.../atoms_01.html
- Atomic Number Review #1: www.sciencegeek.net/Chemistry...micNumbers.htm
- Atomic Number Review #2: www.sciencegeek.net/Chemistry...t1Numbers2.htm
- Atomic Structure: www.sciencegeek.net/Chemistry...omicStructure/
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
 Also found in: Dictionary, Thesaurus, Medical, Acronyms, Wikipedia.
Also found in: Dictionary, Thesaurus, Medical, Acronyms, Wikipedia.atomic mass,
the mass of a single atomatom[Gr.,=uncuttable (indivisible)], basic unit of matter; more properly, the smallest unit of a chemical element having the properties of that element. Structure of the Atom
.....Click the link for more information., usually expressed in atomic mass unitsatomic mass unit
or amu,
in chemistry and physics, unit defined as exactly 1-12 the mass of an atom of carbon-12, the isotope of carbon with six protons and six neutrons in its nucleus. One amu is equal to approximately 1.66 × 10−24 grams.
.....Click the link for more information. (amu). Most of the mass of an atom is concentrated in the protons and neutrons contained in the nucleus. Each proton or neutron weighs about 1 amu, and thus the atomic mass is always very close to the mass numbermass number,
often represented by the symbol A, the total number of nucleons (neutrons and protons) in the nucleus of an atom. All atoms of a chemical element have the same atomic number (number of protons in the nucleus) but may have different mass numbers (from having
.....Click the link for more information. (total number of protons and neutrons in the nucleus). Atoms of an isotopeisotope
, in chemistry and physics, one of two or more atoms having the same atomic number but differing in atomic weight and mass number. The concept of isotope was introduced by F.
.....Click the link for more information. of an elementelement,
in chemistry, a substance that cannot be decomposed into simpler substances by chemical means. A substance such as a compound can be decomposed into its constituent elements by means of a chemical reaction, but no further simplification can be achieved.
.....Click the link for more information. all have the same atomic mass. Atomic masses are usually determined by mass spectrography (see mass spectrographmass spectrograph,
device used to separate electrically charged particles according to their masses; a form of the instrument known as a mass spectrometer is often used to measure the masses of isotopes of elements. J. J. Thomson and F. W. Aston showed (c.
.....Click the link for more information.). They have been determined with great relative accuracy, but their absolute value is less certain.
Atomic mass
The mass of an atom or molecule on a scale where the mass of a carbon-12 (12C) atom is exactly 12.0. The mass of any atom is approximately equal to the total number of its protons and neutrons multiplied by the atomic mass unit, u = 1.660539 × 10-24 gram. (Electrons are much lighter, about 0.0005486 u.) No atom differs from this simple formula by more than 1%, and stable atoms heavier than helium all lie within 0.3%. SeeAtomic mass unit
This simplicity of nature led to the confirmation of the atomic hypothesis—the idea that all matter is composed of atoms, which are identical and chemically indivisible for each chemical element. In 1802, G. E. Fischer noticed that the weights of acids needed to neutralize various bases could be described systematically by assigning relative weights to each of the acids and bases. A few years later, John Dalton proposed an atomic theory in which elements were made up of atoms that combine in simple ways to form molecules.
In reality, nature is more complicated, and the great regularity of atomic masses more revealing. Two fundamental ideas about atomic structure come out of this regularity: that the atomic nucleus is composed of charged protons and uncharged neutrons, and that these particles have approximately equal mass. The number of protons in an atom is called its atomic number, and equals the number of electrons in the neutral atom. The electrons, in turn, determine the chemical properties of the atom. Adding a neutron or two does not change the chemistry (or the name) of an atom, but does give it an atomic mass which is 1 u larger for each added neutron. Such atoms are called isotopes of the element, and their existence was first revealed by careful study of radioactive elements. Most naturally occurring elements are mixtures of isotopes, although a single isotope frequently predominates. Since the proportion of the various isotopes is usually about the same everywhere on Earth, an average atomic mass of an element can be defined, and is called the atomic weight. Atomic weights are routinely used in chemistry in order to determine how much of one chemical will react with a given weight of another. SeeIsotope
In contrast to atomic weights, which can be defined only approximately, atomic masses are exact constants of nature. All atoms of a given isotope are truly identical; they cannot be distinguished by any method. This is known to be true because the quantum mechanics treats identical objects in special ways, and makes predictions that depend on this assumption. One such prediction, the exclusion principle, is the reason that the chemical behavior of atoms with different numbers of electrons is so different.
Atomic Mass
atomic weight; the value of the mass of the atom expressed in atomic mass units. The use of a particular unit for the measurement of the atomic mass is connected with the fact that the masses of atoms are extremely small (10-22 to 10-24g), and to express them in grams is inconvenient. The atomic mass unit is taken as 1/12 of the mass of the carbon isotope12 C. The mass of the carbon unit (CU) is equal to(1.66043 ± 0.00031) x 10-24g. In indicating atomic mass the symbol “CU” is generally omitted.
The concept of atomic mass was introduced by J. Dalton in 1803; he was the first to define atomic mass. A vast amount of work to establish atomic mass was carried out in the first half of the 19th century by J. Berzelius and later by J. S. Stas and T. W. Richards. In 1869, D. I. Mendeleev discovered the law of periodic dependence of the properties of the elements on the atomic mass and on this basis revised the atomic masses of many elements that were well known at that time (Be, U, La, and others), and in addition predicted the atomic masses of the still undiscovered elements Ga, Ge, and Sc. After the discovery by F. Soddy in 1914 of the phenomenon of isotopy, the concept of atomic mass came to be connected both with elements which consist of a mixture of isotopes and with individual isotopes. For elements which are represented in nature by one isotope (for example, F and Al), the atomic mass of the element coincides with the atomic mass of that isotope. If an element is a mixture of isotopes, then its atomic mass is calculated as the average of the values of the atomic masses of the individual isotopes, taking into account the relative percentage of each of them. Thus, natural chlorine consists of the isotopes 35CI (75.53 percent) and 37CI (24.47 percent), the masses of whose atoms are equal to 34.964 and 36.961 respectively. The atomic mass of the element Cl is
The fluctuation of the natural isotopic composition in the majority of elements is negligibly small (less than 0.003 percent); therefore each element has a practically constant atomic mass, which is one of the most important characteristics of the element. The closeness to integers of the atomic masses of elements that are represented in nature by one isotope is explained by the fact that almost all the mass of the atom is contained in its nucleus, and the masses of the protons and neutrons which compose the nucleus are close to I. At the same time, the atomic mass values of the isotopes (other than 12C, whose mass is taken to be 12.00000) are never exactly equal to a whole number. This is explained first by the fact that the relative masses of the neutron and proton are somewhat greater than 1 (1.0086654 and 1.00727663 respectively), second by the mass defect, and third by the small contribution to the total atomic mass of the masses of the electrons.
According to a proposal by J. Dalton (1803), the first unit of atomic mass was the mass of the hydrogen atom (the hydrogen scale). In 1818, Berzelius published a table of atomic masses relative to the atomic mass of oxygen, which was taken to be 100. The Berzelius system of atomic masses predominated until the 1860’s when chemists again adopted the hydrogen scale. In 1906, however, they converted to the oxygen scale, in which the atomic mass unit was taken to be 1/16 of the atomic mass of oxygen. After the discovery of the oxygen isotopes (16O, 17O, 18O) the atomic mass came to be indicated according to two scales—the chemical, based on 1/16 of the average mass of the natural oxygen atom; and the physical, with a mass unit equal to 1/16 of the mass of the l6O atom. The use of two scales had a number of shortcomings; as a consequence, in 1961 there was a conversion to a single (carbon) scale.
Various methods are used for the calculation of atomic mass. Some are based on the experimental determination of the molecular mass of some compound of a given element. In this case the atomic mass is some fraction of the molecular mass of this element divided by the number of the element’s atoms in the molecule. The exact values for atomic mass can be found by determining by chemical analysis the chemical equivalent of the element (the atomic mass is equal to the product of the equivalent times the valence). The atomic mass can be determined with the greatest accuracy (up to 0.001 percent and higher) by the method of mass spectroscopy; the mass spectrum of an element yields information concerning the quantitative isotopic composition and masses of atoms of individual isotopes, on the basis of which it is easy to compute the atomic mass (see example above with 35Cl and 37Cl). The atomic masses of newly synthesized elements are evaluated on the basis of observation of the atomic reaction accompanying their formation.
REFERENCES
Mendeleev, D. I. Osnovy khimii, 13th ed., vols 1–2. Moscow-Leningrad, 1947.Nekrasov, B. V. Osnovy obshchei khimii, vol. 1. Moscow, 1965.
Pauling, L. Obshchaia khimiia. Moscow, 1964. (Translated from English.)
Remy, H. Kurs neorganicheskoi khimii, vol. 1. Moscow, 1963. (Translated from German.)
Gina, M. Istoriia khimii. Moscow, 1966. (Translated from Italian.)
atomic mass
[ə′täm·ik ′mas]:max_bytes(150000):strip_icc()/PeriodicTableoftheElements-5c3648e546e0fb0001ba3a0a.jpg) (physics)
(physics) Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Link to this page:
The Atomic Mass Of An Element Is Equal To __

What Is The Atomic Mass
The Atomic Mass Of An Element Is Equal To The:
